|
|
|
Introduction Neurons
and Neuronal Growth Neurons
and Neuronal Growth
Neurons and Neuronal Signalling
The human brain is a complex network of more than 100 billion interconnected
neurons (from Greek "neuron" for "nerve") [1]. These
extraordinary cells are specialized in information processing and transmission
by electrochemical signalling. A mammalian neuron consists of a cell body,
called soma or perikaryon,
and several cellular processes, called neurites.
Neurites are distinguished into multiple, widely ramified dendrites
and one axon. The latter originates
in the axon hillock and extends over
1 µm to 1 m or even longer, permitting signal transmission over long
distances. The end of an axon is usually connected to dendrites of other
neurons (sometimes to muscle or gland cells) via junctions called synapses.
However, there are also axon-to-soma, axon-to-axon and dendrite-to-dendrite
connections between neurons.
Synapses chemically transmit electrical or electrochemical signals
between the two participating cells by release of neurotransmitters. A
neuron receives the signal with special receptors on the membrane of its
dendrites or the soma. Then, the signal is forwarded to the axon hillock,
where it is decided based on an all-or-none principle if an action potential
is initiated. The action potential travels along the axon as a pulse-like
wave of voltage. This is accomplished by selective ion exchange across
the membrane through voltage-gated ion channels (alteration of the transmembrane
voltage or membrane potential). After reaching the presynaptic side of
another synapse, the signal is passed to the next neuron.
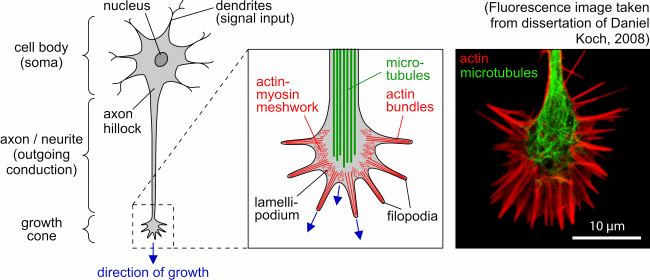
|
| Schematic representation of a neuron and detailed view
of the growth cone with its main cytoskeletal components (actin filaments
and microtubules). The fluorescence image on the right-hand side shows
an NG108 growth cone. (Figure taken from diploma thesis of Steve Pawlizak,
2009.) |
| |
Growth Cones
Developing axons that are not yet synaptically connected have highly
dynamic, motile structures at their leading edge. These structures are
called growth cones. They guide axons to their synaptic target by transducing
positive and negative cues into signals that regulate the cytoskeleton
and thereby determine the course and rate of axonal outgrowth [1,
2].
Hence, growth cones are very important during embryonal and adult neurogenesis.
They are also essential for regeneration of neuronal connections as well
as for the increase of neuronal connectivity.
Growth cones have round or conical shape with two kinds of protrusions:
thin fingerlike filopodia and flat lamellipodia between them. The growth
cone palpates its immediate vicinity with this protrusions and reacts to
attractive or repulsive guidance cues by means of outgrowth, growth cone
turning or retraction. The membrane of growth cones contains special receptors
and cell adhesion molecules that are sensitive to chemical gradients (chemotaxis)
and mechanical substrate properties (durotaxis).
The morphology of growth cones is defined by the underlying structure
of the cytoskeleton. While the axon is dominated by parallel aligned microtubules
forming some kind of backbone, the growth cone mainly consists of actin
filaments. In particular, filopodia contain F-actin bundles and the lamellipodium
a dense F-actin meshwork. The filopodia may act as contractile elements
while exploring their mechanical environment [3, 4].
Prenatal Neurogenesis
Neurons are created through a process called neurogenesis
which mostly occurs during the prenatal development of the nervous system
when the growing brain is populated [5]. The ventricular
zone in the embryonic neural tube
contains progenitor cells [6], which divide in mitotic
cycles, diversify and give rise to neuroblasts
and glioblasts. Eventually, neuroblasts and glioblasts will differentiate
into neurons and glial cells respectively
[7, 8, 1].
At first, radially oriented glial cells are produced, later the neurons
and subsequently all other glial cells. [7, 8].
The radial glia span the thickness of the cortex from the ventricular
zone to the outer, pial surface and provide guiding pathways for the migration
of neurons outwards to their final locations in the gray and white matter
of the nervous system [9, 10].
Once neurons have left the ventricular zone, they become permanently postmitotic,
i.e. they do not divide anymore. On the other hand, glial cells do not
lose their ability to multiply [5,
1].
The distances that neurons travel within the brain are in the range
of several millimeters [9, 11],
whereas cell size is just 10 to 30 µm. The migrating neurons form
well-defined layers whose position is correlated with the date of birth
of the neuron. Inner layers of the cerebral cortex are established first,
outer layer last [1]. After arriving at their destination
sites, neurons differentiate and extend axons, which precisely follow certain
pathways to their connective targets. Within each layer, neurons acquire
distinct morphologies and connections. Radially arrayed neurons in different
layers become richly interconnected and functionally related [12].
When neuronal migration is complete and radial glia are not longer
required as guides, they disappear or transform into astrocytes [13,
14].
Adult Neurogenesis
By the end of the developmental period, the ventricular zone is depleted
of all mitotic cells. However, neurogenesis and neuronal migration are
not limited to embryonic development. It has been shown that even in the
adult brain new neurons are generated [5,
11,
15,
16],
even though the number of new neurons decreases while the organism ages
[17]. This finding disproved the long-held theory
that the nervous system is fixed and incapable of regeneration, but it
does not refute the basic concept that a mature, differentiated neuron
does not divide.
The new neurons originate from neural progenitor cells found in restricted
brain regions, in particular the subventricular
zone (SVZ) lining the lateral ventricles and in the subgranular
zone (SGZ) which is part of the dentate gyrus of hippocampus. The
precursor neurons from the SVZ migrate to the olfactory bulb, where they
differentiate into interneurons. Cells form the SGZ migrate short distances
into the granule cell layer of the dentate gyrus and subsequently differentiate
into granule cells.
It has been reported that limited, localized neuronal injury and hypoxia
induce neurogenesis and replacement of neurons in the adult cerebral cortex
[17].
Neuronal Pathfinding
As already mentioned, both neuronal migration and neurite outgrowth
follow specific pathways. Neurons and growth cones have to detect a variety
of external signals and respond to them accordingly [18,
19,
2,
20].
Neuronal migration and neurite outgrowth are guided by the same molecular
cues [21] with chemotaxis playing a major roll [22,
6,
18,
23].
Several classes of molecules, like diffusible secreted factors, adhesion
molecules and cues from the surrounding extracellular matrix and adjacent
cells are involved in the process of neuronal pathfinding and growth [18,
20,
21].
However, the complex migration and growth patterns of neurons and neurites
cannot be completely explained with simple biochemical gradients, especially
when considering the length of some pathways or the spread of some axons.
In addition to biochemical cues, neurons are susceptible to their mechanical
environment (durotaxis). For instance, enlarged filopodia tips of Aplysia
growth cones are very sensitive to force [24], and
it has been implicated that snail and leech neurons also feel and respond
to external mechanical stimuli [25, 26].
Furthermore, it has been shown that neurite outgrowth and branching in
vitro is influenced by the mechanical stiffness of the substrate [27,
28].
In particular, a preference for soft substrates has been observed. This
characteristic behavior of neurons may be called inverse
durotaxis, since most other cell types (e.g. fibroblasts) favor
stiffer substrates [29, 30].
In vivo, neurons of the CNS grow along glial cells, which are significantly
softer than their neighboring neurons [31]. These
examples suggest an involvement of mechanics in neuronal and axonal guidance.
However, the underlying mechanisms of neuronal mechanosensitivity are not
yet understood.
Directed movement of neurons requires active
recognition of their migration pathway. This motivates us to investigate
the possibly active response of neurons to externally applied mechanical
stress, in order to understand the growth cones’ sensing of mechanical
properties of their environment (e.g. substrate stiffness). Our in vitro
studies show that neurons actively palpate their mechanical environment
with the help of their growth cones and retract their neurites from contacts
they cannot mechanically deform [32]. After mechanical
stimulation of the neuronal growth cones using a modified scanning force
microscope (SFM) probe, the neurons retract their processes and re-extend
them into a new direction when the exerted pressure exceeds approximately
300 Pa. This threshold corresponds to the maximum substrate stiffness that
neurons can visibly deform. Furthermore, an immediate calcium influx through
stretch-activated ion channels seems to be correlated with neurite retraction.
(This article is taken from the diploma thesis of Steve
Pawlizak, University of Leipzig, 2009.)
References:
|
|
|
E. R. Kandel, J. H. Schwartz, T. M. Jessell: Principles
of Neural Science, 4th Edition, McGraw-Hill Medical (2000). |
|
|
|
|
|
|
B. K. Mueller: Growth Cone Guidance: First
Steps Towards a Deeper Understanding, Annu. Rev. Neurosci. 22:351-388
(1999). |
|
|
|
|
|
|
S. R. Heidemann, P. Lamoureux, R. E. Buxbaum: Growth
cone behavior and production of traction force, J. Cell Biol. 111(5
Pt 1):1949-1957 (1990). |
|
|
|
|
|
|
B. T. Schaar, S. K. McConnell: Cytoskeletal
coordination during neuronal migration, Proc. Natl. Acad. Sci. USA
102(38):13652-13657 (2005). |
|
|
|
|
|
|
K. Herrup, Y. Yang: Cell cycle regulation
in the postmitotic neuron: oxymoron or new biology?, Nat. Rev. Neurosci.
8(5):368-378 (2007). |
|
|
|
|
|
|
F. de Castro: Chemotropic Molecules: Guides
for Axonal Pathfinding and Cell Migration During CNS Development,
News Physiol. Sci. 18(3):130-136 (2003). |
|
|
|
|
|
|
S. C. Noctor, A. C. Flint, T. A. Weissman, R. S. Dammerman, A. R. Kriegstein: Neurons
derived from radial glial cells establish radial units in neocortex,
Nature 409:714-720 (2001). |
|
|
|
|
|
|
J. D. Fix: Neuroanatomy (Board Review Series),
4th edition, Lippincott Williams & Wilkins (2007). |
|
|
|
|
|
|
P. Rakic: Mode of cell migration to the
superficial layers of fetal monkey neocortex, J. Comp. Neurol. 145(1):61-83
(1972). |
|
|
|
|
|
|
P. Rakic: Elusive Radial Glial Cells: Historical
and Evolutionary Perspective, Glia 43(1):19-32 (2003). |
|
|
|
|
|
|
A. Alvarez-Buylla, J. M. García-Verdugo: Neurogenesis
in Adult Subventricular Zone, J. Neurosci. 22(3):629-634 (2002) |
|
|
|
|
|
|
M. B. Luskin, A. L. Pearlman, J. R. Sanes: Cell
lineage in the cerebral cortex of the mouse studied in vivo and in vitro
with a recombinant retrovirus, Neuron 1:635-647 (1988). |
|
|
|
|
|
|
J. P. Misson, T. Takahashi, V. S. Caviness Jr: Ontogeny
of radial and other astroglial cells in murine cerebral cortex,
Glia 4:138-148 (1991). |
|
|
|
|
|
|
G. Chanas-Sacre, B. Rogister, G. Moonen, P. Leprince: Radial
glia phenotype: origin,
regulation, and transdifferentiation, J. Neurosci. Res. 61:357-363
(2000). |
|
|
|
|
|
|
E. Gould, A. J. Reeves, M. S. A. Graziano, C. G. Gross: Neurogenesis
in the Neocortex of Adult Primates, Science 286(5439):548-552 (1999). |
|
|
|
|
|
|
N. S. Roy, S. Wang, L. Jiang, J. Kang, A. Benraiss1, C. Harrison-Restelli,
R. A. R. Fraser, W. T. Couldwell, A. Kawaguchi, H. Okano, M. Nedergaard,
S. A. Goldman: In vitro neurogenesis by progenitor
cells isolated from the adult human hippocampus, Nature Medicine
6:271-277 (2000). |
|
|
|
|
|
|
H. T. Ghashghaei, C. Lai, E. S. Anton: Neuronal
migration in the adult brain: are we there yet?, Nat. Rev. Neurosci.
8:141-151 (2007). |
|
|
|
|
|
|
M. Tessier-Lavigne, C. S. Goodman: The
Molecular Biology of Axon Guidance, Science 274(5290):1123-1133
(1996). |
|
|
|
|
|
|
B. J. Dickson: Molecular Mechanisms of
Axon Guidance, Science 298:1959-1964 (2002). |
|
|
|
|
|
|
M. E. Hatten: New Directions in Neuronal
Migration, Science 297(5587):1660-1663 (2002). |
|
|
|
|
|
|
H. T. Park, J. Wu, Y. Rao: Molecular control
of neuronal migration, Bioessays 24(9):821-827 (2002). |
|
|
|
|
|
|
D. Mortimer, T. Fothergill, Z. Pujic, L. J. Richards, G. J. Goodhill: Growth
cone chemotaxis, Trends Neurosci. 31(2):90-98 (2008). |
|
|
|
|
|
|
R. W. Gundersen, J. N. Barrett: Neuronal
Chemotaxis: Chick Dorsal-Root Axons Turn Toward High Concentrations of
Nerve Growth Factor, Science 206(4422):1079-1080 (1979) |
|
|
|
|
|
|
Y. Xiong, A. C. Lee, D. M. Suter, G. U. Lee: Topography
and nanomechanics of live neuronal growth cones analyzed by atomic force
microscopy, Biophys. J. 96:5060-5072 (2009). |
|
|
|
|
|
|
W. J. Sigurdson, C. E. Morris: Stretch-activated
ion channels in growth cones of snail neurons, J. Neurosci. 9(8):2801-2808
(1989). |
|
|
|
|
|
|
B. Calabrese, S. Manzi, M. Pellegrini, M. Pellegrino: Stretch-activated
cation channels of leech neurons: characterization and role in neurite
outgrowth, Eur. J. Neurosci. 11(7):2275-2284 (1999). |
|
|
|
|
|
|
A. P. Balgude, X. Yu, A. Szymanski, R. V. Bellamkonda: Agarose
gel stiffness determines rate of DRG neurite extension in 3D cultures,
Biomaterials 22(10):1077-1084 (2001). |
|
|
|
|
|
|
L.A. Flanagan, Y.-E. Ju, B. Marg, M. Osterfield, P. A. Janmey: Neurite
branching on deformable substrates, Neuroreport 13(18):2411-2415
(2002). |
|
|
|
|
|
|
P. C. Georges, P. A. Janmey: Cell type-specific
response to growth on soft materials, J. Appl. Physiol. 98:1547-1553
(2005). |
|
|
|
|
|
|
C.-M. Lo, H.-B. Wang, M. Dembo, Y. Wang: Cell
Movement Is Guided by the Rigidity of the Substrate, Biophys. J.
79(1):144-152 (2000). |
|
|
|
|
|
|
Y.-B. Lu, K. Franze, G. Seifert, C. Steinhauser, F. Kirchhoff, H. Wolburg,
J. Guck, P. Janmey, E. Q. Wei, J. Käs, A. Reichenbach: Viscoelastic
properties of individual glial cells and neurons in the CNS, Proc.
Natl. Acad. Sci. USA 103(47):17759-17764 (2006). |
|
|
|
|
|
|
K. Franze, J. Gerdelmann, M. Weick, T. Betz, S. Pawlizak, M. Lakadamyali,
J. Bayer, K. Rillich, M. Gögler, Y.-B. Lu, A. Reichenbach, P. Janmey,
J. Käs: Neurite branch retraction is caused
by a threshold-dependent mechanical impact, Biophys. J. 97(7):1883-1890
(2009). |
|
|





