|
|
|||||
|
Introduction The cytoskeleton is a three-dimensional meshwork of entangled, transiently crosslinked biopolymers contained within the cytoplasm of every cell. It was for a long time believed to be a characteristic feature of cells with a nucleus (eukaryotic cells) like all cells from multicellular organisms like fungi, plants and animals. Recently it became clear that even cells without a nucleus (prokaryotic cells) like bacteria have proteins that form a cytoskeleton. In the following, we concentrate on the eukaryotic cytoskeleton. The cytoskeleton provides a mechanical scaffold that stabilizes and protects the cell while also determining its shape. Thus, the cytoskeleton is the major contributor to the mechanical properties of a cell. Nevertheless, it would be inappropriate to think of the cytoskeleton as a completely passive and rigid structure. In fact, it is very dynamic and involved in active cellular processes like cell motion as well as intracellular transport. The cytoskeleton helps the cell to divide during mitosis (cytokinesis) and is responsible for muscular contraction. It seems that the cytoskeleton is also involved in signalling across the cell. The cytoskeleton consists of dynamically assembled and disassembled
protein structures, called filaments.
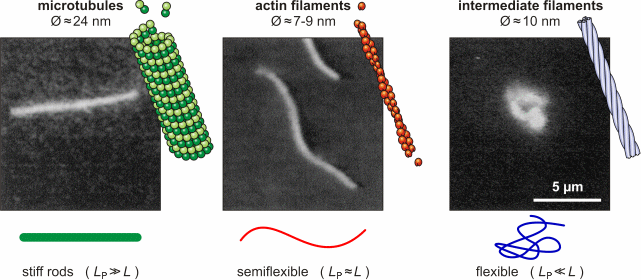
Three main classes of filaments can be distinguished: microtubules, actin
filaments (also called microfilaments) and intermediate
filaments.
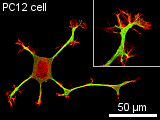
The typical construction in a non-dividing cell in culture is as follows: a thin dense actin-cortex is supporting the plasma-membrane like an underlying shell. Actin stress fibers span through the cell starting from the focal adhesion points, where the cell is attached to the ground. Microtubules radiate from microtubule organizing centers throughout the cytoplasm and are associated with intermediate filaments. The three filament types diversely contribute to the mechanical stability
of the cytoskeleton and the cell, since they differ quite drastically in
their own stiffness. While microtubules mainly resist compression, actin
and intermediate filaments help maintaining the cell-shape by bearing tension.
In terms of polymer physics, the bending stiffness of a filament can be
characterized with help of the worm-like chain
model and the persistence lengthLP.
This concept is introduced in Polymer
Physics.
Microtubules
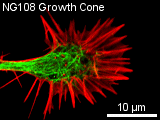
of micrometers. Microtubules consist of two different globular protein subunits, alpha-tubulin and beta-tubulin. One alpha- and one beta-tubulin form a heterodimer. Long linear chains of these heterodimers compose protofilaments, where always a alpha-tubulin is followed by a beta-tubulin. In singlet microtubules generally 13 protofilaments associate site by site forming a hollow cylinder. In some special structures doublet (in cilia and flagella) and even triplet (in centrioles and basal bodies) microtubules exist, having a 13-protofilament microtubule accompanied by one or two 10-protofilament ones. Microtubules assemble and disassemble dependent on the temperature and the surrounding tubulin concentration. Since all heterodimers are arranged in the same direction, microtubules have a polar structure. On one end, designated as (-) end, only alpha-tubulin is exposed and on the opposite end, designated as (+) end, only beta-tubulin is exposed. At the (+) end, new heterodimers are added faster and at lower tubulin concentrations than at the (-) end. When the critical tubulin concentration for the (+) end polymerisation is reached but not the higher critical concentration for the (-) end, the (-) end depolymerises while heterodimers at the (+) end are added. This is called microtubule treadmilling. The alpha-tubulin as well as the beta-tubulin subunit binds a small guanosine triphosphate (GTP). The GTP bound to the alpha-tubulin faces the beta-tubulin subunit of the heterodimer, while the GTP of the beta-tubulin subunit directs away from the heterodimer. When a new dimer is incorporated into a microtubule the beta-tubulin bound GTP is hydrolysed to guanosine diphosphate (GDP). If the polymerisation is faster than the hyrdrolysation, a GTP-cap occupies the (+) end and causes the microtubule to depolymerise faster than polymerise. In a cell the dynamics of microtubules are regulated by microtubule-associated proteins (MAPs). Some MAPs stabilize microtubules, while others destabilize microtubules. Stabilizing MAPs have a microtubule-binding domain and an acidic projection domain, which can bind to intermediate filaments or membranes and is suspected to determine the distance in between bundled microtubules. Such bundles are for example formed in axons with the help of the MAP tau. The ability of MAPs to bind microtubules can be altered by phosphorylation of the MAPs by MAP kinases. Destabilizing MAPs are for example katanin, which breaks bonds between tubulin subunits, or Op18, which increases the disassembly frequency of microtubules presumably by reducing the pool of available heterodimers. The arrangement of microtubules in cells is determined by microtubule-organizing centers (MTOCs). These MTOCs consist of different proteins like gamma-tubulin and pericentrin. The microtubules direct their (-) end to the MTOC. In general every eukaryotic cell has a primary MTOC located near the nucleus and occupied by the centrosome. An important role of microtubules is providing a pathway for intracellular movements of organelles, vesicles and proteins. This is done by motor proteins (kinesins and dyneins) under consumption of adenosine triphosphate (ATP). Most kinesins carry their cargo along microtubules towards the (+) end, while dyneins walk towards the (-) end. These proteins have two head-domains, which are alternating attached to the microtubule and bend before the other head attaches, so that a steady movement along a protofilament is generated. The tail domain of the kinesin appoints the kind of cargo that can be bound and transported. Dynein can bind its cargo not directly to its tail domain but needs the protein dynactin for mediation. Molecular motors may also move microtubules themselves. This is particularly observed in neurons, where microtubules play a significant role for axonal migration by guiding the neuronal growth cone. Furthermore, microtubules perform a special task during mitosis and meiosis by forming the spindle assembly to align and separate the chromosomes.
Actin Filaments
Most multicellular organisms have several actin isoforms. Humans have six actin genes, four encode alpha-actin, one beta- and one gamma-actin. alpha-actin is found in muscle cells where it plays an important role in contracting the cell. beta-actin is localized in the front of moving cells, whereas gamma-actin forms stress fibers. The reversible assembly of actin is the driving force of many cell movements. Nevertheless, F-actin is also involved in intracellular transports. Actin-associated motor proteins, representing the family of myosins, use ATP hydrolysis to generate forces and to walk along the filament. The actin-myosin interaction is responsible for muscular contraction as well as short distance transport (e.g. of vesicles towards the plasma membrane). There is a variaty of proteins that regulate the actin assembly, filament length and stability in vivo. Other proteins promote filament branching. Bivalent cross-linking proteins organize F-actin into networks and bundles. A dense network called the actin cortex is typically found just underneath the plasma membrane. It provides the framework that supports the membrane in order to resist tension und determines the shape of the cell. On the mobile edge of the cell, actin bundles project from the cell to form spikelike filopodia. Between those filopodia, there are lamellipodia formed of actin networks. Special bundles called actin stress fibers span through the cytoplasm. Their terminal sites coincide with the focal adhesion points where the cell is attached to the substrate. Intermediate Filaments Intermediate filaments have no unique structural basis like microtubules
or actin filaments. Instead, they are assembled from a large variety of
different monomeric proteins. Although all these proteins (67 human genes
known) share a common basic structure, they mainly differ at both ends.
The proteins form homo- or heterodimers that further aggregate to tetrameters,
then protofilaments and protofibrils. Finally, four protofibrils associate
laterally to form the ropelike, apolar intermediate filament with a diameter
of 10 nm. Some intermediate filaments are homopolymeres consisting of the
same protein, some are heteropolymeres of two or more proteins.
(This article was written 2003-2007, 2009 by Jens Gerdelmann & Steve Pawlizak.) References:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||