Directed cell migration accompanies us from conception to
death. This integrated process choreographs the morphogenesis of the embryo
during development. The failure of cells to migrate or migration of cell
to inappropriate locations can result in life threatening consequences
such as the congenital defects prominent in the brain. In the adult, cell
migration is central to homeostatic processes such as mounting an effective
immune response and the repair of injured tissues. It contributes to pathologies
including vascular disease, chronic inflammatory diseases, and tumor formation
and metastasis. Understanding cell migration is also becoming important
to emerging areas of biotechnology which focus on cellular transplantation
and the manufacture of artificial tissues. In the following we will discuss
cell motility in Homeostasis as an example of cell motility. Some basic
migration mechanics will be given.
Homeostasis
Immunity and wound healing are two homeostatic processes in the body that
rely on the ability of cells to migrate. Neither of these processes would
be possible if it were not for migration, and often they occur together.
For example, when you cut yourself, the process of wound healing is initiated
to repair the damage. Cells of the immune system are recruited to dispose
of invading bacteria and other microorganisms entering through the wound.
Cells proliferate and migrate to fill the wound. If bacteria opportunistically
entered through the wound, white blood cells (leukocytes) from the circulation
migrate into the surrounding tissue to destroy them. The bacteria are engulfed
by the white blood cells, where potent digestive enzymes destroy them.
Immune cells are constantly on surveillance duty, circulating throughout
the body looking for foreign material to attack and destroy, and thus it
is important for these cells to develop a sense of self so that they can
recognize the bodies own cells and not destroy them. This sense of self
is established early on in their development as they migrate through the
primary lymphoid tissues of the bone marrow and thymus. On the rigth you
can watch a movie of a human polymorphonuclear leukocyte (neutrophil) on
a blood film, crawling among red blood cells, notable for their dark color
and principally spherical shape.The neutrophil is "chasing" Staphylococcus
aureus microorganisms, added to the film.
Basic Migration Mechanics
|
 |
|
|
|
(Figure by Revathi Ananthakrishnan & Allen Ehrlicher,
published in [R. Ananthakrishnan, A. Ehrlicher: The Forces Behind Cell
Movement, Int. J. Biol. Sci. 3:303-317 (2007)]) |
|
|
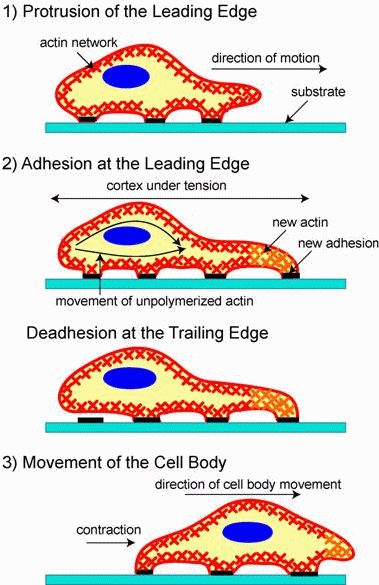
Migration is a dynamic, cyclical process in which a cell extends a protrusion
in direction of motion by actin polymerization at the leading edge (see
figure on the right). It then adheres its leading edge to the surface on
which it is moving via focal adhesions and de-adheres trailing edge of
the cell. Finally, it pulls the whole cell body forward by contracile forces
generated at the cell body and rear of the cell.
This cycle is initiated by external signals (chemotactic molecules),
which are sensed and communicated to the cell's interior by specialized
receptive proteins in the cell membrane. In response to these signals,
cells extend protrusions, by polymerizing actin, that act as feelers, seeking
out new terrain and sensing the direction from which they are receiving
signals. Once the direction for movement is established the machinery for
enabling movement assembles with regard for the direction of migration.
Adhesive complexes needed for traction collect at the front of the protrusion,
tethering the protrusion to the substratum. Actomyosin filaments contract
at the front of the cell and pull the cell body toward the protrusion.
Release of adhesive connections in the rear of the cell and retraction
of the tail completes the cycle. The orchestration of this complex process
resides in many molecules that serve to distinguish the front from the
rear of the cell and whose actions are carefully timed.
This movement process can be observed at a small movie of a crawling
Fibroblasts. The F-actin in the lamellipodia is GFP-Actin fluorescence
labled and thus visible. Click
here.
Parts of this page are copyright to:







