


Through production of fuels and raw materials, people are damaging the environment. More sustainable processes are desirable to reduce this damage. The German word "Nachhaltigkeit" (English: sustainability) was introduced by the mining administrator Hans Carl von Carlowitz in Saxony. His book "Silvicultura Oeconomica" published in 1713 was the first comprehensive treatise about forestry, and he is considered to be the father of sustainable yield forestry.
Sustainable agriculture and forestry can be achieved when living resources are used no faster than their replacements grow back. The concepts of environmental sustainability, economic sustainability, and social sustainability now have many different definitions. The most frequently quoted definition is from the Brundtland Report:
Sustainable development is development that meets the needs of the present
without compromising the ability of future generations to meet their own needs.
It contains within it two key concepts:
Natural resources include land and raw materials like petroleum, coal, and minerals that are mined from the Earth. These were formed over very long geological periods and are non-renewable on human timescales. Thus, they cannot be replenished once they are depleted. Metallic minerals can often be re-used through recycling. But when petroleum, natural gas and coal are burned they cannot be recycled. At current rates of consumption, they will be depleted within the current or next few centuries.
The current extraction, mining, and use of natural resources involve many environmental risks. Assessing environmental risks is not an exact science. There is no doubt, however, that the risks exist and can be explained scientifically. Here, two risks are discussed: the greenhouse effect and radiation hazards from nuclear power plants.
In a greenhouse, the temperature is higher inside than outside. This same effect warms the surface of our planet.
The maximum radiation intensity of the sun is in the green spectral range at about 500 nm wavelength. In this range, the earth’s atmosphere absorbs only a little energy, like the panes in the greenhouse. A greenhouse works by trapping the "green" light from the sun, which is not absorbed by the panes. Solar radiation heats the Earth's surface, the plants, and atmosphere inside the greenhouse by about 10 degrees Celsius above their average temperature. The surface then has a temperature of about 300 K, which is lower than the sun’s surface temperature by a factor of 20. The maximum wavelength of radiation is reciprocally proportional to the temperature of the radiator, according to Wien's displacement law. Therefore, the back reflection from the earth's surface has a wavelength which is longer by a factor of 20 than the light coming from the sun. This reflected radiation is, therefore, in the infrared portion of the spectrum. This infrared radiation is absorbed by glass panes of the glasshouse or by greenhouse gases in the earth’s atmosphere, thus trapping some of the sun’s energy and increasing the temperature of the atmosphere.
The main greenhouse gases (also called global warming gases) are water vapor, carbon dioxide, methane, nitrogen oxides, ozone, and fluorohydrocarbons. The molecules contribute in varying degrees and appear in different concentrations in the atmosphere. For example, a methane molecule causes a 25-fold stronger effect than a carbon dioxide molecule, but methane occurs in relatively low concentrations in the earth’s atmosphere. An estimate of the contribution of individual gases yields in sequence: water vapor, carbon dioxide, methane and ozone, where the relative contributions of the gases are roughly 8, 4, 2, and 1, respectively. Clouds also play an important role and are not accounted for here in the water vapor contribution.
The effect of global warming gases has been studied for many years. The physical chemist Svante Arrhenius (1859−1927) recognized the impact of carbon dioxide as a greenhouse gas and theorized in 1895 that a doubling of the carbon dioxide in the atmosphere would increase the temperature by about 4−6 ºC. At that time, a global warming caused by increasing carbon dioxide in the atmosphere was considered positive for humanity. The concentration of carbon dioxide in the atmosphere can be traced in various ways, one example being to examine ice samples from the deeper layers in the Antarctic. For more than a thousand years, the concentration was 280±10 ppm (parts per million). The value measured in November 2024 was 424 ppm. This means that the carbon dioxide content of the atmosphere has increased by about 50% in less than 200 years. There is also a 145% increase in methane, much of which comes from cows. Nitrogen oxides have increased 15%. It is obvious that most of this growth is due to the burning of fossil fuels for energy.
In the last 50 years, the environment has become of greater public interest. However, the relationship between carbon dioxide emissions and global warming is not much more accurate now than it was in Svante Arrhenius’s time, resulting in low accuracy for climate change forecasts. There are many predictions, both favorable and unfavorable. In 2018, the World Energy Outlook states: "... if the world is serious about meeting its climate targets then, as of today, there needs to be a systematic preference for investment in sustainable energy technologies. ... Under current and planned policies, modeled in the New Policies Scenario, energy demand is set to grow by more than 25% to 2040, requiring more than $2 trillion a year of investment in new energy supply. ... In all cases, governments will have a critical influence in the direction of the future energy system. "
In recent years, predictions about the occurrence and consequences of global warming of two degrees have been the subject of political debate rather than scientific discussion. The public has different views on these predictions. However, there is no doubt that the measures derived from them will serve to accelerate the transition from dwindling non-renewable energy resources to renewable energy sources.
A good transition from discussion of the greenhouse effect (produced by burning fossil fuels) to a discussion of nuclear power plants in the next section is to think about the carbon dioxide emissions that are caused by different plants for electricity production. All plants require a certain amount of energy for construction, operation and deconstruction which today is produced by the combustion of fossil fuels. This contributes to the carbon dioxide input. These contributions show a relatively strong scattering within one type of plant, for example by a factor of four for the photovoltaics. Construction and reconstruction after the life time require a certain amount of energy which determines the carbon dioxide input. The electricity output depends on the location of the installation, since the intensity and duration of sun light is different e.g. between Chicago and Las Vegas. If a photovoltaic installation produces carbon dioxide during its lifecycle, it is not surprising that a nuclear power plant causes carbon dioxide emissions. Values are different by a factor of more the ten and depend on how the enrichment of the uranium for the fuel rods is carried out Compared to the modern laser enrichment, the old diffusion enrichment method, which is still in use today, requires twenty times as much input energy. With the modern enrichment method, however, nuclear energy can compete with some hydropower plants in terms of its carbon footprint. Hydropower plants vary in a much broader range: the best value is 1 g carbonmonoxide per kWh (average over the life cycle including construction and deconstruction), but the mean value is 24 g/kWh.
The Intergovernmental Panel on Climate Change, IPCC, gives in a 2014 report AR5 the following minimum values, it means for the best conditions for each kind of power plant: 740 g/kWh for coal-fired power stations, 410 g/kWh for gas-fired power plants, 18 g/kWh for photovoltaic, 8 g/kWh for solar electric generation systems, 8 g/kWh for wind power offshore, 7 g/kWh for wind power onshore, 4 g/kWh for nuclear power and 1 g/kWh for hydropower. When comparing literature data, however, it should be noted that there is still no standardization of the definition and calculation methods for the carbon footprint, see Wikipedia.
The main environmental concern with nuclear power plants is radioactive contamination. The exposure to radiation during normal operation is quite low, and the major concerns are related to the possibility of catastrophic accidents or terrorist attacks, as well as the final disposal methods of the radioactive waste. Some of these threats are discussed on the page Nuclear Power.
A potential worst-case scenario incident in a light water reactor was described by Professor Kurt Kugler in a report about the safety of nuclear power plants. He detailed in 2001 a case that has strong similarities to what actually occurred in Japan in 2011 (not verbatim): If a light water reactor is deactivated or damaged and there is a simultaneous loss of the residual heat removal, the remaining water in the core area will be vaporized. The reactor fuel and core structures will be heated up and could melt. At the same time, reaction of the zirconium in the fuel rod casing with the steam will produce a large amount of hydrogen and release additional heat from this exothermic reaction. After about an hour under unfavorable conditions, the core can be destroyed, and a 2500 °C hot corium mixture (melted UO2, ZrO2, steel, and fission products) collects in the lower portion of the reactor pressure vessel. Shortly afterward, the hot corium – up to 300 tons – could melt through and crash into the reactor building. This could cause damage to the reactor containment structure. All of this can also lead to a buildup of pressure, which after about four days can cause over-pressurization. A relief valve is in place for this situation. It is designed to send the gas through a filter system and then release it through a stack. Solid fission products and aerosols would be largely retained, with only the gaseous fission products escaping. The resulting hydrogen could lead to an ignition and detonation that might also damage the containment.
Improved safety devices or new procedures are constantly being introduced to reduce risk. However, an accident involving radioactive contamination of the environment cannot be excluded completely.
Another environmental risk comes from the disposal of the spent fuel. Reprocessing the fuel is subject to its own environmental risks. Furthermore the production of nuclear fuel from fresh uranium is still economically cheaper. A different problem arises in countries without nuclear weapons, because reprocessing techniques can be used to obtain weapons-usable plutonium. Nevertheless, reprocessing procedures are under development in part because this is one way to reduce the volume of the waste that must be stored. The complete reprocessing of radioactive waste and transmutation (bombardment with neutrons) of long-life fission products would be the ideal solution, but the corresponding development work has not yet progressed sufficiently.
Thermal power plants usually use the uranium isotope 235. U-235 has a half-life T½ of 700 million years (0.7 × 109 y ) for its radioactive decay, meaning that in 700 million years half of the nuclei have been converted to other isotopes by nuclear reaction. A supernova explosion close to 6 billion years ago produced the following isotopes in approximately equal proportions:
uranium-238 (T½ = 4.5 × 109 y), uranium-235 (T½ = 0.7 × 109 y) and plutonium-239 (T½ = 24 × 103 y).
The Pu-239 decayed very quickly, 0.3% of the U-235 remains, and 40% of the U-238. Uranium fuel assemblies require a U-235 enrichment to at least 3%. During the fission of uranium, U-235 is converted to U-236 by bombardment with slow neutrons. This decomposes immediately to form Ba-139, Kr-94, and three neutrons. The missing mass is converted into energy, see Nuclear Energy. Various radioactive waste products are also produced. For example, the neutron bombardment of the uranium isotope 238 yields 300 kg of the α-emitter plutonium-239 per year of operation for a 1-GW nuclear power plant:

Pu-239 stands at the top of the natural uranium-actinium-decay series and radiates relatively strongly, with a half-life of more than 24,000 years before it converts to U-235, which is much slower decaying and, therefore, less dangerous. Eventually, the nuclei are converted to the stable lead isotope:
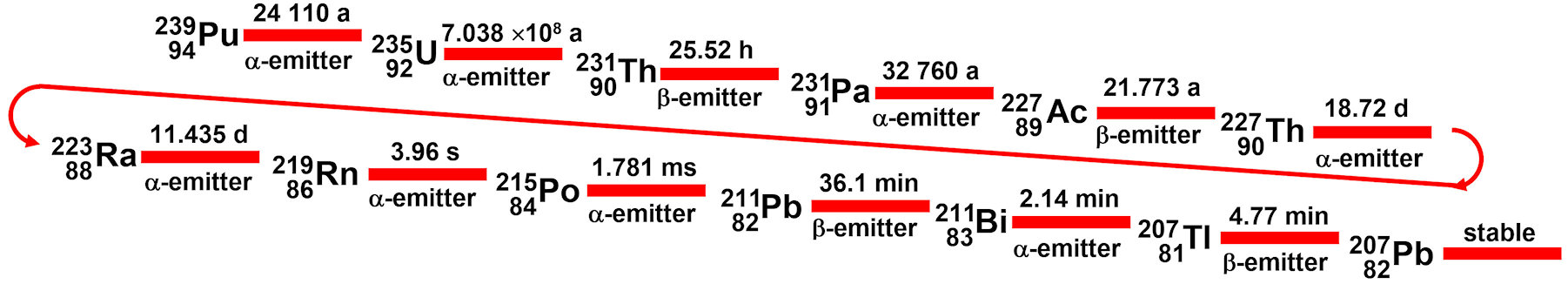
From this discussion, it becomes clear that the final disposal of the waste from nuclear power plants has inherent environmental risks.