

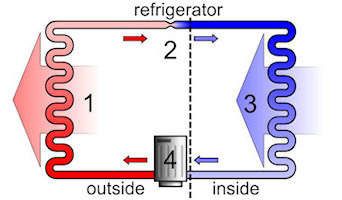
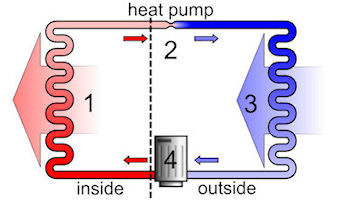
Heat pumps in refrigerators and freezers are found in every household. Heating technicians are increasingly using heat pumps for heating buildings and for energy conservation in various industrial processes. The pictures shown below are two applications. The first picture shows the interior of a refrigerator as it removes heat and releases it to the outside. The second picture shows a heat pump where the heat is pumped from an external medium into the interior of a building.


The energy savings in heating with heat pumps can be explained with the help of the Carnot process. Heat energy for heat pumps is similar to that of the Carnot cycle. Th − Tc is proportional to the mechanical energy required to run the pump. Tc described the temperature of the cooling reservoir and Th is the temperature of the heat reservoir.
The efficiency of heat pumps, η = Th / (Th − Tc), is the reciprocal efficiency of the Carnot process. Therefore, it is much greater than one.
For example, Tc is the temperature of the ground at a length several meters deep; in Chicago, the average temperature is 48 °F ≈ 282 K. A common temperature of under floor heating is Th = 105 °F ≈ 314 K. Then we get, with temperatures in Kelvin, a theoretical efficiency of η = 314 / 32 ≈ 10. But efficiency between 3 and 5 is practical, as all friction losses outside of housing represent heat loss.
In cooling units, special refrigerant agents (also called working fluids) are used. Chlorofluorocarbons have a high ozone depletion potential, and their use is now banned. Propane, Freon and ammonia are other suitable refrigerants. In heat pump systems, water and carbon dioxide also can be used.
Both heating and cooling units operate in a similar fashion. Consider the refrigeration cycle shown above (left). The working fluid, in its gaseous state, is pressurized and circulated through the system by a compressor (4). This requires electrical energy to run the compressor. Then the hot and highly pressurized vapor is cooled in a heat exchanger (1), called a condenser, until it condenses into a high pressure, moderate temperature liquid. The heat is released out from the back of the refrigerator into the surrounding air. The condensed refrigerant then passes through an expansion valve (2). This causes the pressure of the fluid to drop rapidly, and consequently the temperature of the fluid also decreases. The cold, low-pressure, liquid refrigerant then enters another heat exchanger (3), called the evaporator, in which the fluid absorbs heat and boils. The heat for this step is removed from the inside of the refrigerator (causing the refrigerator contents to become colder). The working fluid then returns to the compressor (4), and the cycle is repeated.
Heat pump compressors are electrically operated, while most warm water heaters operate using fossil fuels. It is cheaper to heat with oil or natural gas than with electricity. However, the increased efficiency of the heat pump makes it cheaper, despite the higher cost of electricity. From the other side, the cost of the heat pump system is much higher than in an ordinary heating. There are more details at HPT website.