|
|
|
Introduction Optical
Stretcher & Rotator Optical
Stretcher & Rotator
Optical Stretcher
|
 |
|
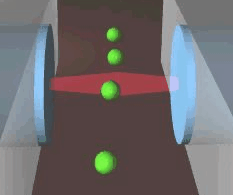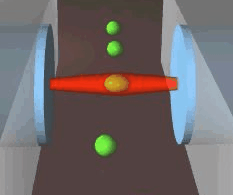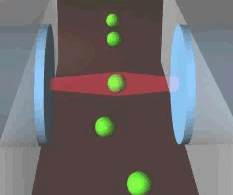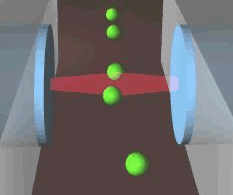
Schematic of an optical stretcher: The Cells are in
suspension in a flow chamber. They can be trapped by two opposing laserbeams
of low intensity, emanating from optical fibers. Raising the intensity
of the laserlight increases the forces acting on the cell surface, leading
to measurable deformation. (Animation by J. Guck et al. [7]) |
|
|
The optical stretcher is a novel laser
tool to micro-manipulate single biological cells and probe their viscoelastic
properties in suspension [1-3].
In the stretcher, an individual cell is trapped between two divergent,
opposing laser beams. A stress is exerted on the cell where the light hits
the surface causing an elongation of the cell body along the laser beam
axis (stress-strain elasticity experiment).
Since reflection (<0.5%) and absorption (<0.01%) of the laser
beam are negligible at the chosen wavelength (780 nm), the laser light
is almost completely transmitted through the cell. The stretching forces
arise from a momentum transfer of light to the surface. The momentum of
the laser beam increases inside the cell because the cell has a higher
refractive index than the surrounding medium. By conservation of momentum,
the beam gives an impulse to the cell, resulting in the stretching force
on the cell surface where the light enters and leaves.
The amount of stretching, or optical deformability, depends on the
force on the surface (which we can be controlled by adjusting the laser
power) as well as the physical properties of the cell, such as size and
refraction index.
The stretching forces (pN to nN) exerted by the optical stretcher can
be up to 100 times higher than the holding forces for optical tweezers
without causing any radiation damage, since the two laser beams are not
focused and the optical stretcher does not rely on gradient forces.
Combined with a microfluidic flow chamber, the processing of large
numbers of cells is possible, which is a big advantage over conventional
techniques for measuring cell elasticity. (Those techniques are often limited
due to laborious sample preparation).
We have derived an analytical model to calculate the stress profile
on the cell's surface. This model has been verified by stretching objects
with well-defined elasticity.
The response of the cells to the applied stress profile can be well
described by a viscoelastic three-parameter model, and reveals a lot of
information about the material properties of the cell.
 |
| Comparison between the deformation of a red blood cell
observed in the optical stretcher and the deformations expected from membrane
theory (white lines) showing an excellent agreement. The peak stresses
sigma_0 calculated using ray optics were used for the membrane theory calculations.
(Figure taken from [2].) |
| |
Application of the Optical Stretcher for
Cancer Diagnosis
Using an optical stretcher, the cell elasticity can be accurately measured.
This material property of cells can be used to differentiate between different
cell types or between normal and unhealthy cells.
Since changes in the cytoskeleton are characteristic in the pathology
of cancer, we investigated whether single malignant cells and precancerous
cells can be detected by elasticity measurements with the optical stretcher.
Model cell lines were used to explore to what extent cell elasticity is
a good parameter to detect cancer cells. We compared fibroblasts to clonal
populations of these cells malignantly transformed by H-ras, SV40, or v-rel
and normal neutrophils to leukemia cell lines. It turned out that malignant
cells generelly are easier to stretch and show a lower elastic strength
[7, 10, 13].
The ultimate goal of these cell elasticity studies with the optical
stretcher is to distinguish dysplastic cells from early cancer cells and
to monitor the progress of cancer from preinvasive to invasive. We also
investigate whether we are able to separate pluripotent stem cells from
blood stem cells in umbilical cord blood based on cell elasticity. This
could open new routes in stem cell research avoiding the ethical problems
with stem cells from embryos. It is planned to have a clinical device for
the early diagnostics of cancer on the market within the next 5 years.
Optical Cell Rotator
|
 |
|
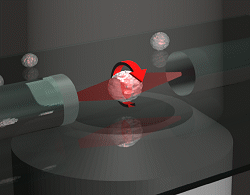
Schematic of an optical rotator, which is is mounted
on an inverted microscope. The suspended cells are trapped and rotated
by two opposing laserbeams of low intensity, emanating from optical fibers.
(Figure by Anatol Fritsch, 2009.) |
|
|
One inherent problem of optical tomography, for example when using an confocal
laser scanning microscope, is its resolution in axial direction. The lateral
resolution exceeds the axial at least by a factor of 2 to 3. Rotation of
the object of interest along a horizontal axis can resolve this issue and
the higher lateral resolution for every rotation angle could be used to
image the desired cell-detail and eventually recalculate an isoresolution
3D image.
The optical cell rotator is a modified
divergent dual-beam laser trap for holding and controlled rotation of suspended
cells [14]. Cell align with their long axis inside
this optical trap when using two gaussian beam profiles like in the optical
stretcher. With the optical rotator, an asymmetric beam is introduced on
one side of the trap. Since cells are naturally inhomogeneous, they align
somehow to this beam profile. When now the asymmetric beam profile is rotated,
a rotation of the trapped cell itself is induced. Since the optical rotator
is fully decoupled from imaging optics, it could be a beneficial tool for
tomographic microscopy.
 |
| A cluster of several MCF-7 cells is rotated by 360
degrees. This videos already allow a good impression of the 3D topology
of the cells. (Figure by Anatol Fritsch & Tobias Kießling, 2009.) |
References:
|
|
|
J. Guck, R. Ananthakrishnan, T. J. Moon, C. C. Cunningham, J. Käs: Optical
Deformability of Soft Biological Dielectrics, Phys. Rev. Lett. 84(23):5451-5454
(2000) |
|
|
|
|
|
|
J. Guck, R. Ananthakrishnan, H. Mahmood, T. J. Moon, C. C. Cunningham,
J. Käs: The Optical Stretcher: A Novel
Laser Tool to Micromanipulate Cells, Biophys. J. 81(2):767-784 (2001) |
|
|
|
|
|
|
J. Guck, R. Ananthakrishnan, C. C. Cunningham, J. Käs: Stretching
biological cells with light, J. Phys.: Condens. Matter 14(19):4843-4856
(2002) |
|
|
|
|
|
|
B. Lincoln, H. M. Erickson, S. Schinkinger, F. Wottawah, D. Mitchell,
S. Ulvick, C. Bilby, J. Guck: Deformability-based
flow cytometry, Cytometry Part A 59A(2):203-209 (2004) |
|
|
|
|
|
|
F. Wottawah, S. Schinkinger, B. Lincoln, S. Ebert, K. Müller,
F. Sauer, K. Travis, J. Guck: Characterizing
single suspended cells by optorheology, Acta Biomaterialia 1(3):263-271
(2005) |
|
|
|
|
|
|
R. Ananthakrishnan, J. Guck, F. Wottawah, S. Schinkinger, B. Lincoln,
M. Romeyke, J. Käs: Modelling the structural
response of an eukaryotic cell in the optical stretcher, Current
Science 88(9):1434-1440 (2005) |
|
|
|
|
|
|
J. Guck, S. Schinkinger, B. Lincoln, F. Wottawah, S. Ebert, M. Romeyke,
D. Lenz, H. M. Erickson, R. Ananthakrishnan, D. Mitchell, J. Käs,
S. Ulvick, C. Bilby: Optical Deformability
as an Inherent Cell Marker for Testing Malignant Transformation and Metastatic
Competence, Biophys. J. 88(5):3689-3698 (2005) |
|
|
|
|
|
|
F. Wottawah, S. Schinkinger, B. Lincoln, R. Ananthakrishnan, M. Romeyke,
J. Guck, J. Käs: Optical Rheology of Biological
Cells, Phys. Rev. Lett. 94(9):98103 (2005) |
|
|
|
|
|
|
R. Ananthakrishnan, J. Guck, F. Wottawah, S. Schinkinger, B. Lincoln,
M. Romeyke, T. Moon, J. Käs: Quantifying
the contribution of actin networks to the elastic strength of fibroblasts,
Journal of Theoretical Biology 242(2):502-516 (2006) |
|
|
|
|
|
|
M. Martin, K. Mueller, F. Wottawah, S. Schinkinger, B. Lincoln, M.
Romeyke, J. A. Käs: Feeling with light
for cancer, Proceedings of SPIE 6080:126-135 (2006) |
|
|
|
|
|
|
B. Lincoln, S. Schinkinger, K. Travis, F. Wottawah, S. Ebert, F. Sauer,
J. Guck: Reconfigurable microfluidic integration
of a dual-beam laser trap with biomedical applications, Biomedical
Microdevices 9(5):703-710 (2007) |
|
|
|
|
|
|
S. Ebert, K. Travis, B. Lincoln, J. Guck: Fluorescence
ratio thermometry in a microfluidic dual-beam laser trap, Optics
Express 15(23):15493-15499 (2007) |
|
|
|
|
|
|
T. W. Remmerbach, F. Wottawah, J. Dietrich, B. Lincoln, C. Wittekind,
J. Guck: Oral Cancer Diagnosis by Mechanical
Phenotyping, Cancer Research 69(5):1728-1732 (2009) |
|
|
|
|
|
|
M. K. Kreysing, T. Kießling, A. Fritsch, C. Dietrich, J. R. Guck,
J. A. Käs: The optical cell rotator,
Optics Express 16(21):16984-16992 (2008) |
|
|








