P5: Diffusion and Conformational Dynamics in Locally Perturbed Model Membrane Systems
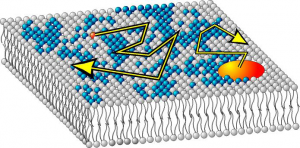 Anomalous diffusion in cell membranes is an intriguing phenomenon still lacking a proper understanding and clear explanation based on the molecular picture. From the general viewpoint, the phenomenon is related to (dynamic) microheterogeneities in the properties of the cell membrane creating a rugged energy landscape for the diffusing protein or lipid molecule. The current understanding of membrane microdomains has evolved from two competing concepts of lipid rafts and cytoskeleton-based picket fence. Nowadays, the concept of membrane rafts implies a dynamic interplay between the membrane local composition and phase, and the local membrane-biopolymer interactions, which can lead to the local lipid demixing and phase separation. This viewpoint was beautifully confirmed by the exciting new results we obtained during the previous funding period. In particular, we discovered a new unexpected phenomenon of DNA condensation at free-standing cationic lipid bilayers (manuscript in revision). Our hypothesis is that this effect is related to the deformation-assisted lipid demixing, and thus can be controlled by changing the membrane bendability. We believe that varying the tension in membranes with or without phase separation will allow us to explore different modes of DNA interaction with charged membranes, and to get a better understanding of this exciting phenomenon. Further, our Monte Carlo simulation studies of diffusion in membranes close to the phase transition have unexpectedly revealed the presence of transient anomalous diffusion of lipid molecules that may be related to results reported previously in experiments (manuscript in preparation). Using our simulation approach, we will check the hypothesis that the membrane interaction with the cytoskeleton further enhances the anomalous diffusion and additionally affects the phase transition behavior of the lipid membrane. To check the above hypotheses experimentally, we will reconstitute artificial actin networks on model lipid membranes (both supported and free-standing) and will experimentally study the diffusion and phase separation in these locally perturbed model membrane systems. Our results will contribute to a better understanding of the organization and dynamics of cell membranes and their interaction with proteins and cytoskeleton.
Anomalous diffusion in cell membranes is an intriguing phenomenon still lacking a proper understanding and clear explanation based on the molecular picture. From the general viewpoint, the phenomenon is related to (dynamic) microheterogeneities in the properties of the cell membrane creating a rugged energy landscape for the diffusing protein or lipid molecule. The current understanding of membrane microdomains has evolved from two competing concepts of lipid rafts and cytoskeleton-based picket fence. Nowadays, the concept of membrane rafts implies a dynamic interplay between the membrane local composition and phase, and the local membrane-biopolymer interactions, which can lead to the local lipid demixing and phase separation. This viewpoint was beautifully confirmed by the exciting new results we obtained during the previous funding period. In particular, we discovered a new unexpected phenomenon of DNA condensation at free-standing cationic lipid bilayers (manuscript in revision). Our hypothesis is that this effect is related to the deformation-assisted lipid demixing, and thus can be controlled by changing the membrane bendability. We believe that varying the tension in membranes with or without phase separation will allow us to explore different modes of DNA interaction with charged membranes, and to get a better understanding of this exciting phenomenon. Further, our Monte Carlo simulation studies of diffusion in membranes close to the phase transition have unexpectedly revealed the presence of transient anomalous diffusion of lipid molecules that may be related to results reported previously in experiments (manuscript in preparation). Using our simulation approach, we will check the hypothesis that the membrane interaction with the cytoskeleton further enhances the anomalous diffusion and additionally affects the phase transition behavior of the lipid membrane. To check the above hypotheses experimentally, we will reconstitute artificial actin networks on model lipid membranes (both supported and free-standing) and will experimentally study the diffusion and phase separation in these locally perturbed model membrane systems. Our results will contribute to a better understanding of the organization and dynamics of cell membranes and their interaction with proteins and cytoskeleton.
Project Leaders
Prof. Dr. Petra Schwille
Dr. Eugene Petrov
Max Planck Institute of Biochemistry
Department of Cellular and Molecular Biophysics